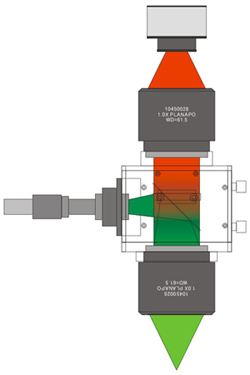
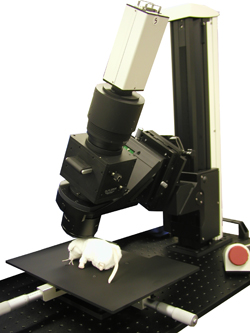
The THT is a fluorescence widefield macroscope developed for detecting low light fluorescence that is emitted from biological sample at a low magnicifation. This macroscope has a very simple optical path combining two large-diameter objective lenses and a custom-made large fluorescent filter.
Custom-made systems can be constructed according to your request.
Low Magnifications with High N.A Values:
Detecting Low Light Fluorescence with a Wide Field of View
Depending on the combination of lenses, the total magnification is about 0.19x to 6.3x (Actual field of view varies depending on the sensor size of the camera used).
Because a large diameter and high numerical aperture lens is used, extremely bright imaging is possible. Signals that were previously difficult to detect may be easier to detect, and even samples that could be captured so far may have higher S/N ratios.
| Objective Magnifications |
Lens Diameter | Working Distance |
|---|---|---|
| 0.3x | 50mm | 140mm |
| 0.63x | 43mm | 67mm |
| 1x | 60mm | 62mm |
| 1.6x | 59mm | 31mm |
| 2x | 57mm | 20mm |
| 5x | 39mm | 19mm |
| Projection Lens |
Magnification | Mount |
|---|---|---|
| 50mm | 0.63x | C |
| 85mm/1.4 | 1.0x | EF/T/C |
| 135mm/2.0 | 1.6x | EF/T/C |
Simple Structure: Specializing in Imaging
There is no visual observation function. It is designed to focus on camera shooting with a higher S/N ratio.
The optical axis can be tilted and rotated: no need to tilt the sample
A stand that can rotate the optical axis 360 degrees left and right is also selectable. By tilting the optical axis, it is possible to image the entire temporal lobe of the brain without tilting the animal sample.
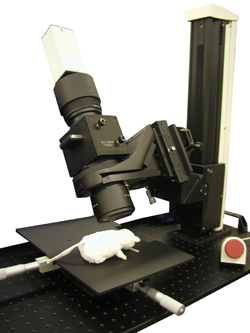
Easy Removal of Fluorescent Filter
The fluorescent filter is held in a removable filter cube. the fluorescent filter can be changed by replacing the filter cube.
Multifunction: Used As a Fluorescence Beam Splitter
Normally, an excitation light source is connected to the left port of the THT main unit and used as a coaxial epi-illuminator.
In addition, it can be used as a two-wavelength fluorescence splitting device. In this case, the same lens and camera as the upper port are connected to the left port, and two-wavelength fluorescence simultaneous measurement using two cameras is performed.
Inverted type is also possible
An inverted fluorescence macroscope has been manufactured. The space on the sample stage becomes freer, so we expect that the degree of freedom in manipulator installation and electrode operation will increase. It can also be used with an upright fluorescent microscope. Please feel free to ask us for microscope bases and sample stages to suit the purpose of the experiment.
Applications
- Membrane potential imaging using voltage-sensitive dyes
- Widefield calcium imaging
- Intrinsic optical signal (IOS) imaging
- Imaging FRET, GCaMP, and various fluorescent proteins such as GEVI
- Ratiometric fluorescence imaging with multiple camera heads

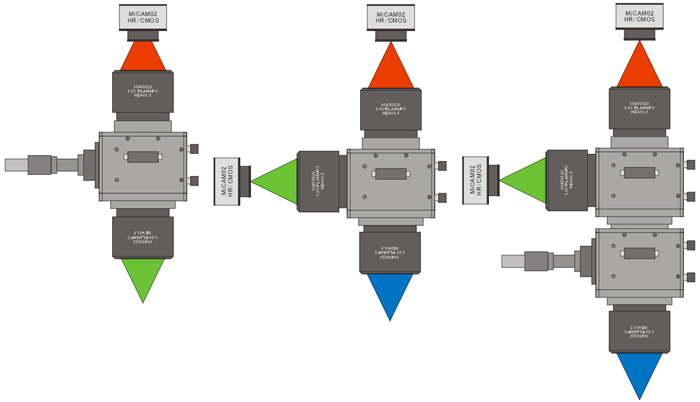
Epi illumination/single wavelength imaging

Side illumination/dual wavelength imaging

Epi and side illumination
dual wavelength imaging, Upright

Epi and side illumination
dual-wavelength imaging, 90 degrees rotation

Epi-illumination
Single wavelength imaging
Brainvision XY stage included

Olympus BX51WI base
includes manual XY table from Luigs&Neumann, Germany
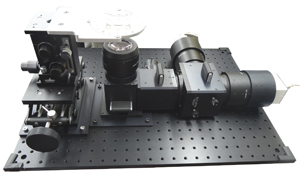
Interted/dual wavelength imaging

Horizontal imaging for Langendorff perfused heart

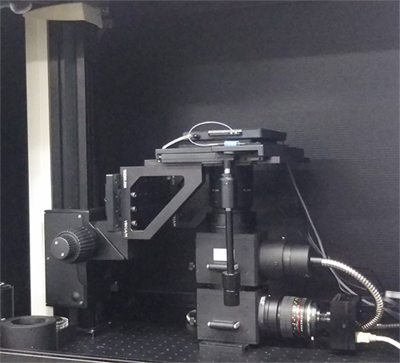
High rigid arm type for In Vivo imaging
| Model | Fluorescence Macroscope THT Series |
|---|---|
| Optical System | Tandem lens optical system used for camera only |
| Focusing device | Manual coarse/fine focus drive or Motorized coarse/fine focus drive (incl. dial type controller) |
| Microscope carrier | Objective nosepiece (two M65 ports), or tilting mount |
| Filter Cube | Detachable One 50mm diameter filter can be installed on the top and side Dichroic mirror mount with 2-axis tilt adjustment mechanism |
| Illumination Light for Excitation | Coaxial epi-illumination or side illumination |
| Lens port | Top: 1, Side: 1, Bottom: 1 |
| Objective lens | PLAN 0.3x PLAN APO 0.63x PLAN APO 1x PLAN APO 1.6x PLAN APO 2x PLAN APO 5x |
| Projection lens | PLAN APO 0.63x PLAN APO 1x PLAN APO 1.6x PLAN APO 2x 135mm/2.0 lens (w/ aperture and focus adjustment function) 85mm/1.4 lens (w/ aperture and focus adjustment function) 50mm/0.95 lens(w/ aperture and focus adjustment function) |
| Sample stage | Option |
Approximate magnification and field size for N256 camera and THT series
| Objective Lens |
Condensing Lens (Camera Side) | |||||
|---|---|---|---|---|---|---|
| 0.63x | 1x | 1.6x | 50mm (∞ setting) |
85mm (∞ setting) |
135mm (∞ setting) |
|
| 0.3x (WD:141mm) |
0.48x (36.6mmx36.6mm) |
0.3x (58.6mmx58.6mm) |
0.19x (92.6mmx92.6mm) |
– | – | – |
| 0.63x (WD:67mm) |
1x (17.6mmx17.6mm) |
0.63x (27.9mmx27.9mm) |
0.4x (44mmx44mm) |
0.4x (44mmx44mm) |
0.69x (25.5mmx25.5mm) |
1.27x (13.9mmx13.9mm) |
| 1x (WD:61.5mm) |
1.6x (11mmx11mm) |
1x (17.6mmx17.6mm) |
0.63x (27.9mmx27.9mm) |
0.64x (27.5mmx27.5mm) |
– | 2x (8.8mmx8.8mm) |
| 1.6x (WD:30.5mm) |
2.6x (6.7mmx6.7mm) |
1.6x (11mmx11mm) |
1x (17.6mmx17.6mm) |
1.02x (17.6mmx17.6mm) |
– | – |
| 2x (WD:20.1mm) |
3.2x (5.5mmx5.5mm) |
2x (8.8mmx8.8mm) |
1.26x (13.9mmx13.9mm) |
1.28x (13.7mmx13.7mm) |
2.23x (7.9mmx7.9mm) |
4x (4.4mmx4.4mm) |
| 5x (WD:19mm) |
6.3x (2.8mmx2.8mm) |
4x (4.4mmx4.4mm) |
2.5x (7mmx7mm) |
– | 4.48x (3.9mmx3.9mm) |
– |
THT Macroscope is used for GCaMP imaging.
Chronic multiscale imaging of neuronal activity in the awake common marmoset.
Yoshiyuki Yamada, Yoshifumi Matsumoto, Norio Okahara & Katsuhiko Mikoshiba
Scientific Reports volume 6, Article number: 35722 (2016)
Cortical State Fluctuations during Sensory Decision Making.
Elina A.K. Jacobs, Nicholas A. Steinmetz, Andrew J. Peters, Matteo Carandini, Kenneth D. Harris
Curr Biol. 2020 Dec 21;30(24):4944-4955.e7.
Cellular and Widefield Imaging of Sound Frequency Organization in Primary and Higher Order Fields of the Mouse Auditory Cortex.
Sandra Romero, Ariel E Hight, Kameron K Clayton, Jennifer Resnik, Ross S Williamson, Kenneth E Hancock, and Daniel B Polley
Cereb Cortex. 2020 Mar; 30(3): 1603–1622.